Latest Posts
Long Carbon Nanotubes Could Have Carcinogenic Effect Similar to Asbestos
Nanomaterials in consumer products
The increase on nanotechnology research has brought along a wide variety of products containing nanomaterials (NMs) already placed in the market. In a inventory from the Project on Emerging Nanotechnologies at the Woodrow Wilson International Center of Scholars, over 1,000 nano-enabled products are listed, and the most common NMs used are nanoscale silver, carbon, titanium, silicon, zinc and gold.
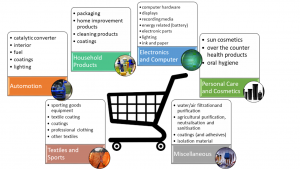
From these consumer products, the group of coatings and adhesives contains the highest amount of nanomaterials, mainly polymers in aqueous suspension for the applications in e.g. exterior paints, coatings and adhesives and in the finishing of textiles and leathers. Other product groups that are ranked high on basis of the number of NMs is the group of food packaging materials, catalytic converters, automotive components and UV absorbers.
However, the exact number of products containing NMs and the amount of NMs embedded or released from everyday products used in the total global consumer market is still an unknown, mainly due to the fact that there is no legal obligation to label products that contain nanomaterials, except in cosmetics and medicine, and more recently food and biocides. Therefore, some manufacturers might be incorporating them from intermediates without knowledge.
The European chemical legislation requires manufacturers and importers to do consumer exposure assessment when the chemical has certain hazards proved, but due to the uncertainty and technical challenges related to exposure and risk assessment of NMs, there is still a lack of data, especially when referring to the consumer exposure.
The way in which consumers are exposed to substances can generally be characterized by:
- The different routes of exposure, separately or in combination. These are predominately inhalation, dermal and oral routes.
- The identification of the different phases of activity in handling, use and disposal of the product or article,
- The duration and frequency of exposure.
Depending on the nanomaterial, its physical state and the matrix in which is embedded, the routes of exposure can be different.
Consumer exposure: open questions
The potential health risks of consumer products containing NMs depend on the possible hazards of the NMs in these products and the likely of exposure to these nanomaterials. However, it is difficult to assess both factors due to different issues:
- There is no systematic approach for evaluating potential consumer risks.
On one side, the potential for human exposure under realistic exposure and use scenarios is not well characterized. Likehood and magnitude of exposure can range from short-term to life-time depending on the product, and the exposed population will change over the product lifecycle . Likewise, the relevant routes of entry into the body are multiple and, contrarily to the occupational exposure where the inhalation route is the most probable, is not clear the relevance of the dermal and oral routes due to the integration of the NMs within a matrix.
The same problems affect the toxicological studies, where is not clear whether in-vivo or in-vitro essays are realistic or the doses applied are representative enough.
- A major challenge is the identification of the products and the NMs contained on them. The lack of obligation to label the nano-components in a product derives in the inclusion of NMs in the fabrication chain of a product, and the unawareness of the production quantity, value, chemical entity and concentration of the specific NMs. Besides, the constant increase of nanotechnological applications involves a large and rapidly growing number of possible NMs to be tested.
...If it contains nanoparticles? Uhh... I don´t know, have you read the nano-label?
- The scarcity of exposure assessment studies and lack of monitoring are keeping risk assessors from conducting comprehensive studies of nanomaterials. Products can potentially release nanomaterials into the environment during the manufacturing process, use/misuse, and disposal.
Which products contain NMs?
Since manufactures are not required to report the use of NMs (except CNTs by the EPA - SNUR directive of September 2010), the methodology for identifying consumer products containing nanomaterials consists of collecting information from various sources:
- Existing databases, from which the most popular is The Nanodatabase, developed by the Department of Environmental Engineering of the DTU (Denmark). It contains more than 3000 products and is frequently updated.
Another source is the PEN inventory , which relies on self-identified products and may thus potentially over or understate the number of commercial ‘nanoproducts’.
The RIVM from Holland also developed an inventory of products containing NMs with more than 200 entries.
The most recent relative only to cosmetic and wellness products is the "Catalogue of nanomaterials used in cosmetic products placed on the market" from the EU Comission, released on 12/07/2017 and subject to modifications and regular updates.
- Information from manufacturers, although the most important limitation to include the product on the public inventories is that is not verified if the products actually do contain NMs, since it is based on claims of manufacturers and no measurements were conducted on these products.
- Legal Directives. In the European framework, the horizontal legislation such as REACH (EC 1907/2006) covers the NMs by substance definition, but the registration is currently not explicitly mentioned within REACH. Similar occurs with Regulation (EC) No 1272/2008 on Classification, Labelling and Packaging (CLP) of dangerous substance, where the inclusion of NMs is yet undetermined. Some other initiatives have been launched, such as:
- Regulation (EC) N° 1223/2009 for finished cosmetic products when placed on the EU market. It reinforces product safety while taking into consideration the latest technological developments, including the possible use of nanomaterials
- Regulation (EU) No 1169/2011 on the provision of food information to consumers, introducing the requirement of a list of engineered nanomaterials in the ingredients.
- Regulation (EU) No 528/2012 on biocide products (BPR), where the approval of active substances does not generally cover the nanomaterial form of active substance - separate dossier needed.

In the U.S., GAO concluded, “FDAs approach to regulating ENMs allows ENMs to enter the food supply as GRAS substances without FDAs knowledge”, while in 2010, CPSC staff produced an annual report on the overall use of NMs in the marketplace and the consumer product categories that contain NMs.
- Information from literature, mostly on analytical measurements of products or properties. A comprehensive recent compilation can be found here.
Determinants of exposure
Several factors determine the level of exposure to consumers; though there is no consensus yet on which are they and on how extent influence the exposure. Many properties of the NMs required for the risk assessment are in common with the occupational and environmental approaches. Nevertheless, specific properties regarding the final product that reaches consumers must be selected and grouped depending on the sector of application. Then, specific properties related with each of the exposure routes.
The most important characteristics for categorisation of the exposure to NMs, besides the chemical entity and the shape of the NM in the consumer product, are:
- Whether the NM is free or fixed inside the matrix of the consumer product: it is likely that exposure is low or negligible when the NM not expected to be able to migrate, leach, evaporate or escape from the matrix
- Concentration levels of the NM in the product. Unfortunately, this information is almost always unknown o can vary drastically with usage time.
- The way of usage or application of the product. whether application is expected to lead to direct exposure to NMs or to indirect exposure via release of NMs out of the product to the surrounding environment. This fact determines factors such as how, how much, how long and by how many people is affected by the use of the product.
- The route of exposure (via inhalation, ingestion or via skin) is an important characteristic. In general, inhalation is generally expected to lead to higher internal exposure levels than dermal exposures, although exposure may occasionally and potentially occur via other routes such as eyes (splashing) or intradermal (e.g. by earrings or piercings).
How to assess the impact?
The risk assessment of consumer products generally does not cover the nanomaterial form of the active substance. Therefore, a dedicated risk assessment is needed, besides form a risk-independent labelling of the nano-ingredients.
Currently, due to the lack of a standardized methodology to evaluate the risk and the difficulties previously stated, the most common way to assess the exposure to consumers is through models.
However, many models of exposure are either not specific for NMs, but are appropriate for consumer exposure (ECETOC TRA, Consexpo, ESIG…), or consider nanomaterial properties but are not specific for consumer exposure (Nanosafer, NF/FF…). A summary of the available models can be found in the following table:
Table 1: Description and classification of the most relevant models that cover consumer exposure.
| Consumer model | Consider NMs? |
Exposure Route |
||
| (y/n) | oral | dermal | Inhalation | |
| Precautionary Matrix | Y | x | x | x |
| NanoRiskCat / Nano-Database | Y | x | x | x |
| DeRmal Exposure Assessment Method (DREAM) | N | x | ||
| ECETOC Targeted Risk Assessment (TRA) v.3 | N | x | x | x |
| Consumer GES/CSA tool | N | x | x | |
| British Aerosol Manufacturers’ Association (BAMA) Indoor Air Model | N | x | ||
| NanoSafer 1.0/1.1 | Y | x | ||
| A.I.S.E. REACT Consumer tool | N | x | x | x |
| Near Field/Far Field model | Y | x | ||
| ConsExpo | N | x | x | x |
| ConsExpo nano | Y | x | ||
>




